|
The main research fields are
briefly described as follow:
(1)
Potential-resolved Electrochemiluminescence
(2)
Electrochemiluminescence on Nanoparticle
self-assembled Electrodes
(3)
Liquid Phase Chemiluminescence Induced by Gold
Nanoparticles
(4)
Exploration of New chemiluminescence systems
(5)
Enhancement and Inhibition
effects of Chemiluminescence
1. Potential-resolved Electrochemiluminescence
|
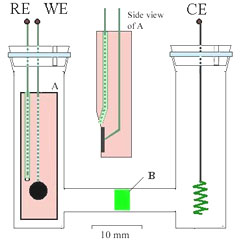
Electrochemiluminescence cell assembly |
Under cyclic voltammetric conditions,
several electrochemiluminescece (ECL) channels can be
resolved at different potentials for conventional ECL
systems, which is referred to as potential-resolved
electrochemiluminescence (PRECL). However, ECL initiated
by electropulse signals cannot resolve these channels.
The PRECL behaviors of typical chemiluminescence
systems, such as luminol, lucigenin and
tris(2,2¡¯-bipyridyl) ruthenium, on various electrodes
(including Pt, Au, Cu, glass carbon,
paraffin-impregnated graphite, and Ti rod electrodes)
have been systematically investigated. It reveals that
the ECL of luminol, lucigenin and tris(2,2¡¯-bipyridyl)
ruthenium exhibit multi-channel emission, which depends
on the electrode material, the applied potential and the
surface state of the electrodes. Furthermore, the
mechanisms of each channel have also discussed.
The potential-resolved
electrochemiluminescence has opened a new ground for the
research of electrochemiluminescence. It is not only of
great importance for probing into the mechanism of ECL
and exploiting new ECL systems, but also of great
potential for developing highly sensitive and selective
ECL analytical methods.
|
|
The papers about the research
mentioned above have published on Anal. Chem., J.
Electroanal. Chem., and so on.
Reviewers commented: ¡°Future
interesting development of this work, in analytical
science, would consist in possible multi-channel light
emissions.¡± Related work had orally reported on the
12th International Symposium on Bioluminescence and
Chemiluminescence.
H. Cui, G.Z. Zou, X.Q. Lin
¡± Electrochemiluminescence of luminol in alkaline
solution at a paraffin-impregnated graphite electrode ¡±
Anal. Chem. 2003, 75(3), 324-331.
|
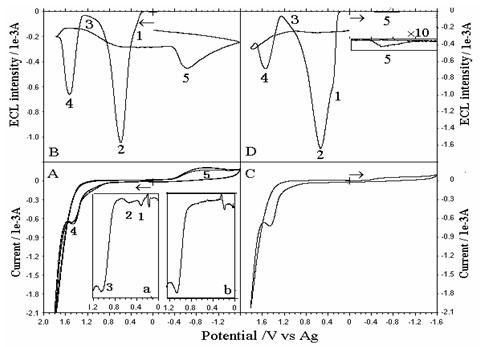
Compare PRECL curve with CV curve of luminol on
paraffin-impregnated graphite electrode |
Top |
|
¡¡ |
|
¡¡
2. Electrochemiluminescence on Nanoparticle
self-assembled Electrodes
|
Previous
work has revealed that the behavior of luminol ECL is
strongly affected by a number of factors, including the
applied potential, electrode material, and surface state
of the electrode. These results indicate that it is
expected that ECL-based
detection can be improved through
optimization of the composition and surface structure of
the electrode. Nanoparticle self-assembled electrodes
have received considerable attention in electrochemistry
and electroanalytical chemistry. Various metal, metal
oxide and nonmetal oxide nanoparticles or clusters have
assembled on different electrodes, including Au, Pt, Ag,
C and so on. These self-assembled electrodes exhibit
fascinating surface-absorption, molecule-identifying,
electrocatalytic properties and high reactivity.
However, the study concerning ECL on nanoparticle
self-assembled electrodes has not been reported until
now. |

CCD image of luminol ECL on a gold nanoparticle
self-assembled electrode |
|
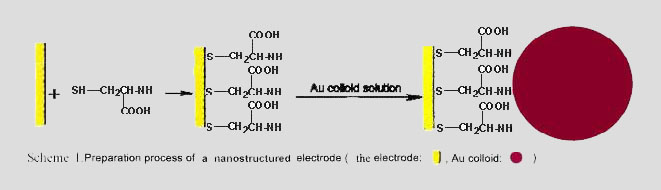
Scheme
of preparation of gold electrode modified with gold
nanoparticles |
H. Cui, Y. Xu, Z.F. Zhang
¡± Multi-channel electrochemiluminescence of luminol in
neutral and alkaline aqueous solutions on a gold
nanoparticles self-assembled electrode ¡±
Anal. Chem., 2004, 76(14): 4002-4010 |
¡¡
|
Recently, our group has assembled
gold nanoparticles on various conventional electrodes,
and explored the ECL behaviors of luminol and lucigenin
on these gold nanoparticle self-assembled electrodes. It
was found that the light intensity was greatly enhanced;
new emission channels appeared; stability and
reproducibility were largely improved, which did not
exist on conventional electrodes. These results show
that the sensitivity of luminol and lucigenin detection
system can be largely improved on gold nanoparticle
self-assembled electrodes.
This work is not only of great
importance for enriching our knowledge on the unique
properties of microscaled substances, but also of great
potential for practical application of nanotechnology in
analytical chemistry.
Top |
¡¡ |
|
¡¡ |
|
¡¡
3. Liquid Phase Chemiluminescence Induced by Gold
Nanoparticles
|
Beautiful CL in liquid
phase |
The
special optical properties of nanoparticles contain a
plenty of information of their energy level structure
and surface states, and therefore, it attracts more and
more researchers. At present, the research about the
optical properties of nanoparticles mainly includes the
surface plasmon resonance absorption (SPR), surface
enhanced raman spectroscopy (SERS), and the
photo-generated luminescence, etc.
Chemiluminescence is the light emission phenomenon go
with the chemical reaction. It played an important role
in the research field of thermodynamics, kinetics, and
light emission property of physical and chemical
process. Since Albrecht observed the CL phenomenon of
luminol in alkaline solution, several liquid-phase CL
system were widely studied and developed, including
luminol and its derivatives, acridinium ester,
bis(2,4,6-trichlorphenyl)oxalate (TCPO), acidic
potassium permanganate (KMnO4), ruthenium(II)
polypyridine (Ru(bpy)32+), and
Ce(IV), etc. However, the study of liquid-phase
chemiluminescence often focused on the molecular and
atomic level, or simple congeries, such as micelles and
microemulsions. In recent years, the study of CL and ECL
behaviour of semiconductor nanoparticles attracted many
groups' eyes. |
|
Recently, we found gold that nanoparticles could induce
the liquid-phase CL reaction, playing important roles as
both the reduction reagent and the catalyst. For
example, the gold colloid could have a reaction in
KIO4-NaOH/Na2CO3
system and generate a strong chemiluminescence.
According to our present analysis, the three CL peaks of
its CL spectroscopy are corresponded to emission of
singlet oxygen (490-500nm), CO2 bi-molecules
(430-450nm) and Au(I) intermediate, respectively (See
detailed information on J.
Phys. Chem. B. 2005, 109, 3099). In the other work, we
found the catalysis effect of gold nanoparticles to
luminol chemiluminescent reaction, and its good
potential for analytical application.
It is believed that this
work expanded the CL object from molecule, ion systems
to metal nanoparticles. |
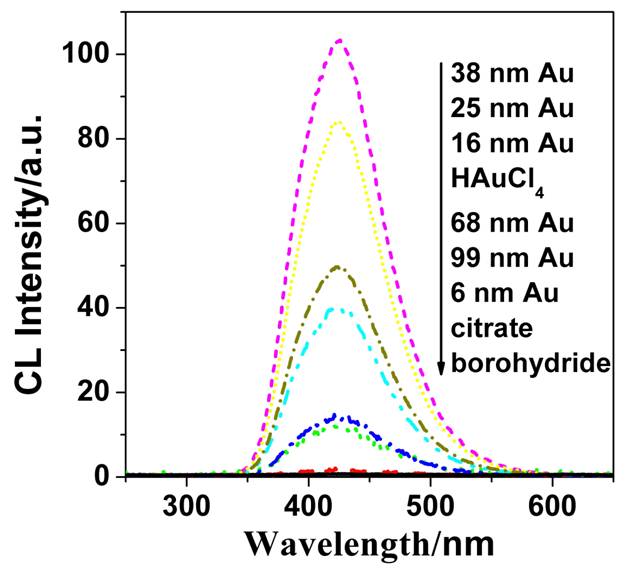
Luminol-H2O2
CL spectroscopy of different particles radius |
Top |
|
¡¡ |
|
¡¡
4. Exploration of New chemiluminescence systems
It is
well known that lots of present chemiluminescent reactions have low
quantum yields and are difficult to have practical analytical
applications, although there are many system for chemiluminescence.
Therefore, there is a great significance for analysts to explore new
chemiluminescent system. We found
Ce(IV) + Tween20 and Ce(IV) +
rhodamine 6G + phenolic compounds systems, proposed their reaction
mechanisms, and established a high sensitivity CL detection method
for many phenolic compounds and flavonic compounds.
|
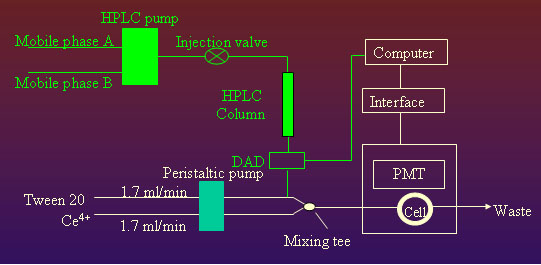 |
The
flowing chart for HPLC-CL analysis of Ce(IV) + Tween20
system.
(see left) |
|
The
chromatography curve of a practical sample of HPLC-CL
detection of
Ce(IV) +Tween20
system. (see right) |
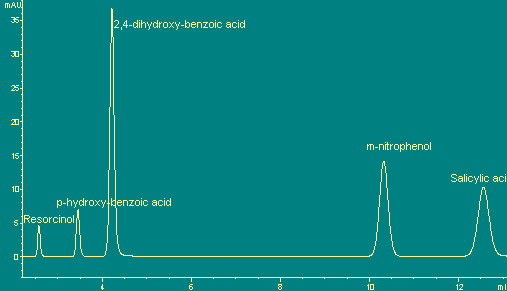 |
It is believed that
this work will have a bright future for the practical analytical
applicaiton in drug analysis, food safety determination, and
monitoring of environment. The first paper was published on "Analyst".
The record from Royal Society of Chemistry demonstrated that the
click number of this paper was top 3 in May, 2001.
Top |
|
¡¡ |
|
¡¡
5. Enhancement and Inhibition effects of Chemiluminescence
The linear
relationship between the concentration of analyte and the
corresponding CL/ECL strength can be used as a quantitative
analytical tool. However, the proper systems for CL/ECL are
limited, which greatly limited the application of CL/ECL
analysis method. In this case, the enhancement and inhibition
effects for different compounds to the same or few CL/ECL system
play an important role in the analytical determination.
Unfortunately, the rules in CL enhancement and inhibition
effects are still not clear.
Therefore, we had a systematic study about the influence of a
series of phenolic and aniline compounds, and amino acids on
many CL systems, such as luminol-K3[Fe(CN)6],
luminol-H2O2-Co(II), luminol-DMSO-OH-,
luminol-H2O2-IO4-,
lucigenin-H2O2-Co(II), Ce(IV)-Tween 20,
etc, and ECL systems, such as luminol,
Ru(bpy)32+/C2O42-,
Ru(bpy)32+/TPA, etc, in different conditions.
Based on these experimental results, we found the rules of
enhancement and inhibition effects are greatly dependent on the
molecular structure, concentration, and the pH of solution.
What's more, the mechanisms of these
phenomenon were also proposed. And then, the corresponding
flow-injection with CL detection method and the HPLC-CL method
of these compounds were established. Furthermore, we discovered
a new CL system, i.e.,
Ce
(IV) + Tween 20, proposed the reaction
mechanism, and established the high sensitivity
CL analysis to many phenolic and flavonic
compounds.
Top |







